- Atoms are so small that it’s almost impossible to see them without microscopes.
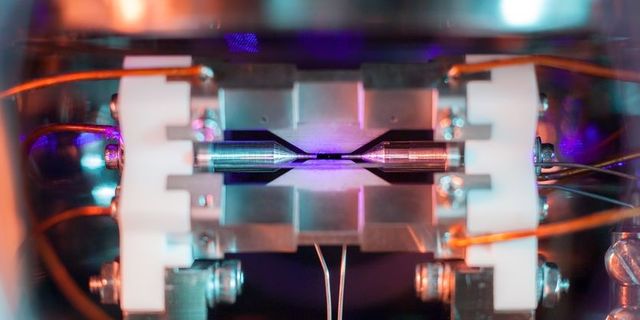
- But now, an award-winning picture of an atom shows a single particle in an electric field—and you can see it with the naked eye if you really look hard.
- This is a strontium atom, which has 38 protons; the diameter of a strontium atom is a few millionths of a millimeter.
Atoms are really small. So small, in fact, that it’s impossible to see one with the naked eye, even with the most powerful of microscopes. At least, that used to be true. Now, we have a picture of an atom that shows the particle floating in an electric field, and it’s large enough to see with the naked eye.
Taken by David Nadlinger and titled “Single Atom In An Ion Trap,” the photo at the top of this story is the winner of the Engineering and Physical Sciences Research Council’s 2018 science photography competition. The photo depicts a single strontium atom, embedded inside a strong electric field, blasted by lasers which cause it to emit light.
⚛️ Know Your Terms: An atom is the basic building block of matter, according to the U.S. Nuclear Regulatory Commission. Each atom is made up of smaller subatomic particles: protons, neutrons, and electrons. Protons have a positive charge, electrons have a negative charge, and neutrons—as their name suggests—have no charge.
The center of an atom, the nucleus, is composed of both protons and neutrons; the number of protons in the nucleus determines an atom’s “atomic number,” and therefore, its place on the Periodic Table. The protons in the nucleus also give rise to an atom’s characteristics.
Even though the atom is visible, it’s still not the easiest to see. If you look very closely at the center of the photo, you’ll see a faint blue dot. That’s the strontium atom, illuminated by a blue-violet laser.
This particular apparatus uses strontium because of its size: strontium has 38 protons, and the diameter of one of these atoms is a few millionths of a millimeter. Normally this would still be much too small to see, but this setup employs a clever trick to make the atom much brighter.
The strontium atom in the photo is hit by a high-powered laser, which causes the electrons orbiting the strontium atom to become more energized. Occasionally, these energized electrons will give off light. With enough energized electrons giving off enough light, it’s possible for an ordinary camera to image the atom.
Still, that doesn’t mean you’ll be able to see the atom with your own eyes. This picture of an atom is a long-exposure shot, which means even with all that laser light, it’s still too faint to pick up without equipment. But given how incredibly tiny atoms are, looking at this photo is probably the closest you’re going to get.