Getting anything down to true absolute zero—where there is no atomic movement whatsoever—is probably a physical impossibility. But in our quest to see how cold things can get, we've come across some pretty unusual states of matter.
PBS SpaceTime gets down to the molecular level for a look.
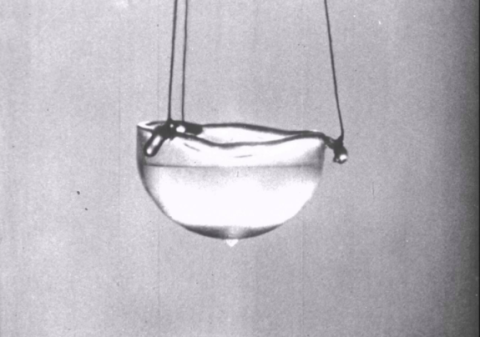
Helium is a unique element, a bit of a loner on the periodic table in terms in how it can handle the cold. Helium-4, an isotope of helium, can remain liquid right down to the fabled absolute zero and, in fact, changes into a substance known as a superfluid. Heat is friction, atoms bumping into each other. But taken down to its coldest levels, a helium-4 superfluid knows no frictional boundaries.
Only discovered in 1983, the helium superfluid allows some for some of the weirder aspects of quantum physics to become visible to the naked eye. If you shook a cup filled with superfluid helium, according to a professor speaking to Scientific American, the liquid wouldn't settle even after a million years.
Creating endless amounts of superfluids isn't the main reason the United States has a helium reserve, but perhaps it should be.
Source: PBS SpaceTime
David Grossman is a staff writer for PopularMechanics.com. He's previously written for The Verge, Rolling Stone, The New Republic and several other publications. He's based out of Brooklyn.