Carbon is quite an abundant element. It's the basis of life on Earth, and comes in a variety of forms. Its unique chemical structure makes it readily bond with other atoms to form molecules, whether with other elements or itself.
One of those forms, cyclocarbons, form a perfect ring. They've been teased out in the lab before, but only in gaseous states that quickly wore away. They could potentially have some interesting properties if we could find a way to work with them. But cyclocarbons are tricky. They're really volatile— so reactive, in fact, that studying them has proven to be a fool's errand.
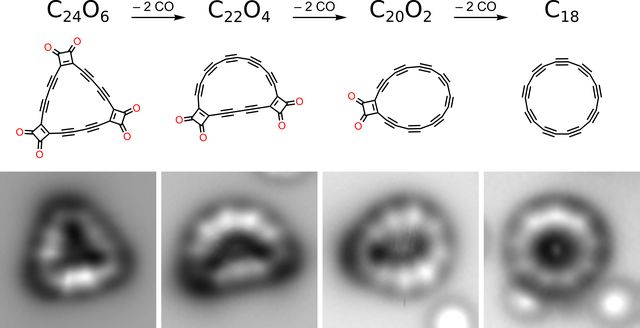
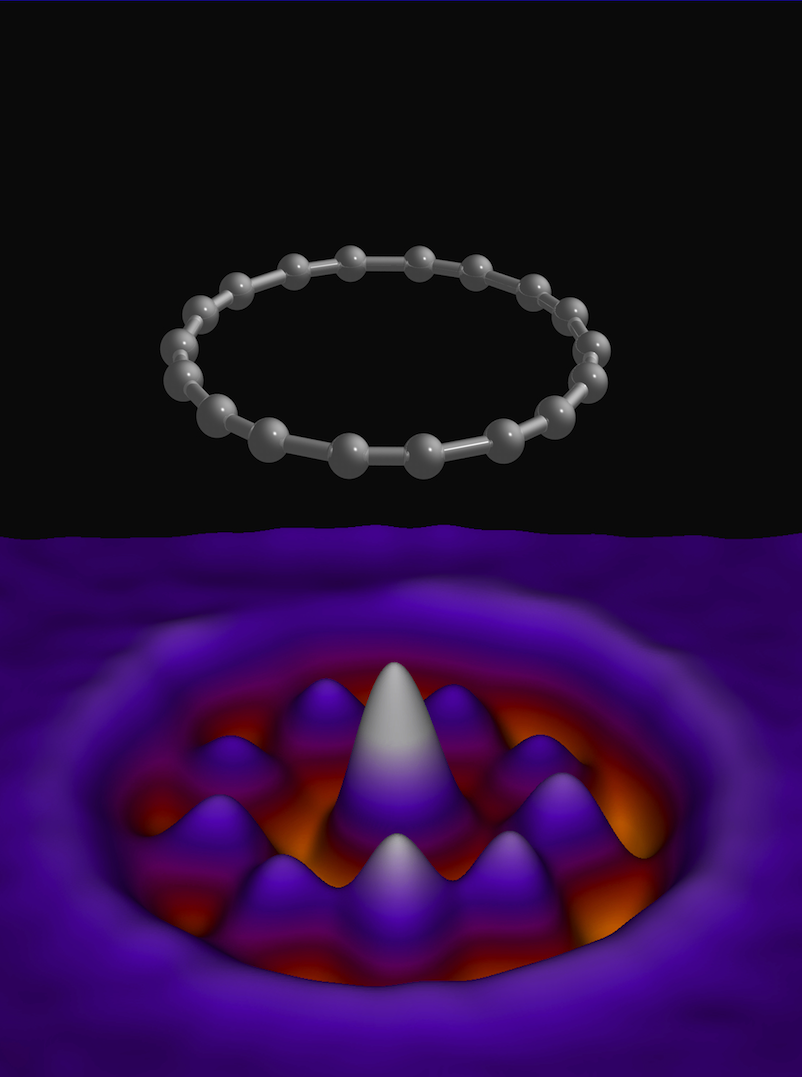
But research published in Science today changes that. For the first time, a cyclocarbon— known as cyclo[18]carbon, or 18-carbon—has been created in the lab in a solid form, and it all came down to the manipulation of carbon at the atomic level.
The Carbon Conundrum
First the researchers at IBM Research and Oxford took a molecule of 24 carbon atoms linked with six oxygen atoms, placing it in sodium on a copper surface kept just a few degrees above absolute zero. That caused it to emit off carbon monoxide atoms one by one until there were 18 carbon atoms left, each interconnected and sharing electron shells.
And all of this was captured by an electron microscope, meaning the researchers actually saw it in action.
Initially, the team had thought of trying to arrange it atom-by-atom, a capability that has only come in the last few years. But they couldn't arrive at the right precursors to do this, arriving instead at the method used. Previously, carbon-oxygen bonded molecules had been suggested as a possible method for synthesis, stretching back at least 30 years. It helped fulfill a decades-long quest to synthesize a cyclocarbon.
"They are built of a ‘naked’ carbon atoms that are additionally bent out of their optimal linear geometry," Przemyslaw Gawel of Oxford University, an author on the paper, says. "Therefore, they could never be stabilized enough to clearly characterize them in experiment."
Nancy Goroff, a professor of chemistry of Stony Brook University, called it a "beautiful culmination of work," allowing the manipulation of the cyclocarbons, which had been detected previously in only trace amounts. "In matrix-isolation studies in the 1990's, we were able to demonstrate that the carbon oxides lose carbon monoxide when exposed to high-intensity light at low temperatures, but we could not image the resulting cyclocarbons," she said in a statement sent to reporters.
Breaking Down Carbon
Carbon, as the sixth element, has six electrons, two in an inner shell and four in an outer shell. That shell can accommodate up to 8 electrons, so there's plenty of room for carbon atoms to bond. This is part of why it so readily makes other molecules, but also why some forms — including most cyclocarbons — are unstable. They're prone to create new compounds or otherwise react into other forms of carbon.
Because of this reactivity, researchers are still figuring out what they can do with the cyclocarbons now that they've successfully synthesized them. The team admits that it's early in the process to have an immediate use, but right now they're thinking they can use the reactivity of the chemicals to their advantage. IBM Research is already manipulating atoms.
"At some point we might be able to controllably trigger such reactions and create more complex molecular structures at will," Katharina Kaiser of IBM Research and a coauthor on the paper says.
That means that, while cyclocarbons on their own are short-lived, it could be a building block to more and more complex structures. Chemists could build new carbon chains at the molecular level—after all, atomically thin carbon is already out there in forms like nanotubes and graphene, which have incredible electronic applications.
Using a ring of carbon, researchers could custom build compounds with properties not yet explored.
The Future of Electronics
And one area where carbon shows a lot of promise is in future electronic applications. Other atomic-scale carbon, like graphene, has shown excellent conductivity with electrons hitting relativistic speeds.
It's too early to tell if it will be able to conduct electrons at quite those speeds, but Leo Gross, an IBM Research staff member and coauthor on the paper, says initial tests are promising, and that future devices made with 18-carbon as a basis could also be quite power efficient.
One area they may be applied? Physical neural networks, which would power artificial intelligence "brains" in complex decision making skills by modeling the outcome of a series of solutions to various problems. Most "neural networks" today are simulations of physical neural networks relying on a series of algorithms. But the researchers think they could use it to physically model neurons—an idea that stretches back to the beginnings of artificial intelligence research.
Neural nets, whether physical or virtual, rely on overlapping processes connected together. To work on the same problem, they need to be able to make conclusions rapidly based on other interconnected processes—much like our own actions throughout the day are a complex aggregate of simple decisions.
Mini-electronics built with cyclocarbon components could be physically connected together at the atomic level—tiny carbon neurons that create a true artificial brain.
"We are looking for devices that can have a large number of connections between each other and in which the strength of these connections can be tuned," Leo Gross, an IBM Research staff member and coauthor on the paper, says. This sort of device mimics the neural network of our own brain.
"In addition the devices should be extremely power efficient, like our brain is."
But that's getting a bit ahead of things. After all, they've just now isolated it for the first time. Now they can start testing what it can really do.